常州江苏大学工程技术研究院
Changzhou Engineering and Technology Institute of Jiangsu University
Welcome to Changzhou Engineering and Technology Institute of Jiangsu University!
International Cooperation

Aptamer Based Biosensing
Project background
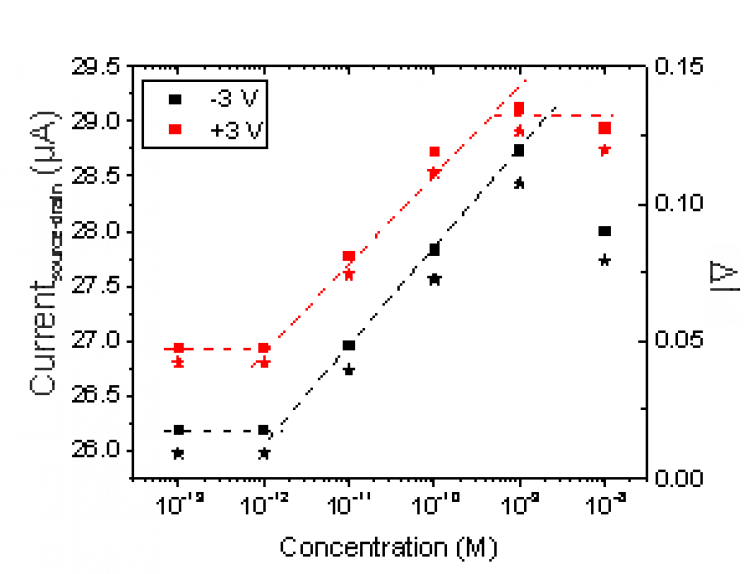
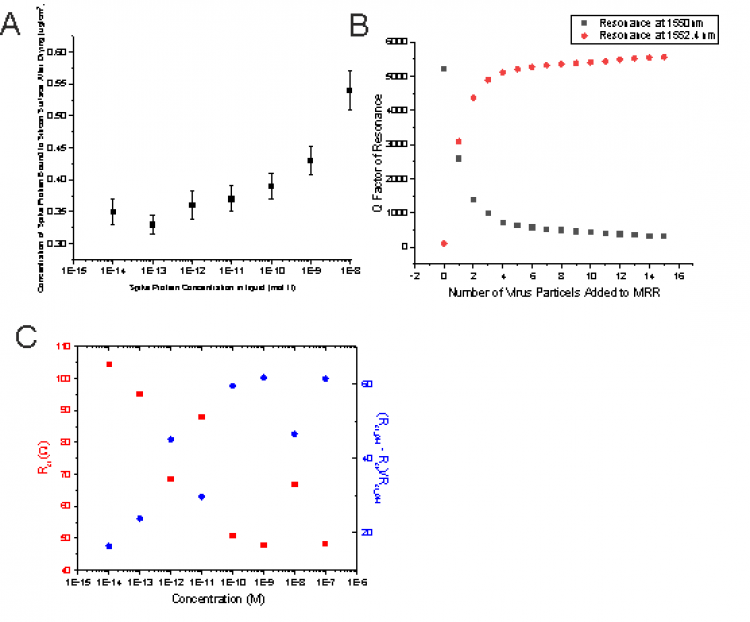
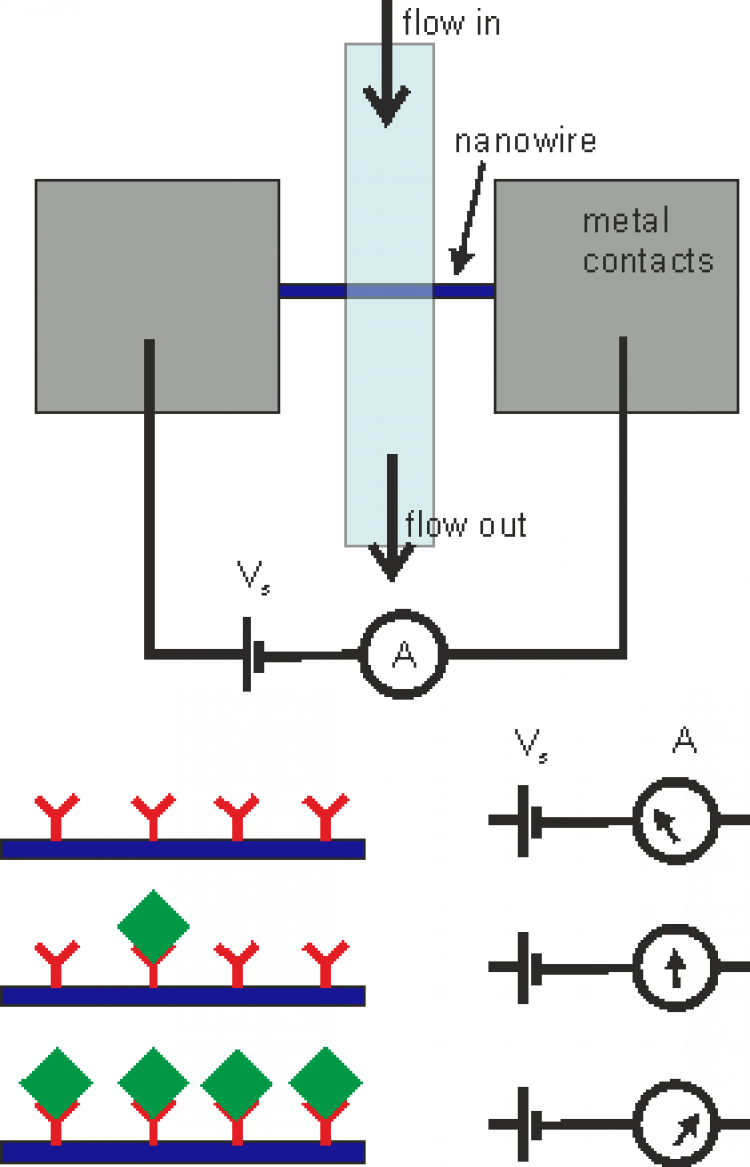
We are developing a sensory platform capable of rapid, i.e. less than 5 minutes, detection of a wide range of potential targets (viruses, toxins, proteins, etc.). Recently, we have successfully used field-effect transistors functionalized with an aptamer to detect the spike protein associated with SARS-Cov-2 and have also achieved similar results for the detection of vascular endothelial growth factor (VEGF). The use of aptamers is a novel approach, which enables the target molecule to reside much closer to the sensor than in conventional antibody-based sensors. The close proximity provides higher sensitivity, while aptamers have similar selectivity as antibodies. Long term the aim is to develop a range of rapid diagnosis tests suitable for at home / in community use for the detection of single targets as well as a more comprehensive analytical tool capable of screening for multiple targets simultaneously. We have so far realized aptamer-based sensors using a number of different approaches including; electrical methods (Si, ZnO and nanowires transistors), optically (via micro ring resonators and fluorescence based techniques), and using electrochemical approaches (impedance spectroscopy). Using a wide range of approaches enables us to better understand the aptamer-target interaction and obtain an optimum approach for each required target.
Technology summary
Recently a number of so-called lab-on-a-chip bio-sensors have been demonstrated for the detection of a wide range of target proteins and molecules (including viruses, toxins). While these sensors have been realized on a range of platforms (electrical, optical, etc.) they typically work by using an antibody to attach the target protein to the sensor. The selectivity in these devices is created using the antibody while the sensitivity can be enhanced by varying the dimensions of the active area. Currently a major restriction for the use of antibody-based biosensors is the size of the antibodies, which is similar to that of the proteins/ molecules that they are aimed to detect. This results in a relatively small change in the physical parameter that is being studied. In our work we are looking to utilize aptamers instead of antibodies. Aptamers consist of short DNA, RNA or peptide strands. Utilising their conformation and charge distribution the aptamers are capable of very specific binding to individual proteins. As they are significantly smaller than antibodies, the result is that a protein bound via an aptamer will create a larger change in signal level.
Benefits/Advantages
One of the key advantages to using aptamers for bio-sensors instead of more traditional antibodies is their much smaller size. This means that it becomes possible to use this system with physiologically relevant liquids and helps to obtain a higher sensitivity. In addition, aptamers are significantly cheaper and more stable than antibodies resulting in lower cost and longer lifetimes for the end sensors. By investigating a range of sensing techniques (electrical, optical and electrochemical) we will be able to optimize the sensor for any given application. This approach will also enable us to potentially incorporate multiple detection strategies on the same device to enhance the performance (provide less false positive/negative results).
Market application
In general, it can be extrapolated that if an aptamer can be found that specifically binds to any required target an aptamer-based sensor is feasible. We foresee two potential markets for these sensors: 1. Rapid, low cost sensing for use by individuals at home /community settings. A single sensor would be used for a particular target with no need for trained medical personal (in a similar way to current lateral flow / pregnancy tests). 2. Medical diagnosis – by developing a multiplexed array of sensors, each with different aptamers (for different targets) a wide range of possible conditions, diseases can be tested for simultaneously from a single sample (blood, saliva, urine, etc.) While this approach would be more complex and expensive, likely requiring some medical / technical knowledge it would still provide a step change in current medical diagnosis by being able to screen for the presence of multiple diseases simultaneously.
Ways of collaboration
Joint technical collaboration – We are looking for potential collaborators and partners who may be able to help advance this work. Particular areas we are interested in are: 1) Aptamer Discovery Techniques (to enable more target protein / molecules to be detected), 2) End Users (i.e. health care for potential trials, feedback, discussion) 3) Semiconductor Material Growth/Development to enable other possible materials to be tested as transistor/resonators for sensing (i.e. Graphene, GaO, Polymers, etc)
· Technology commercialization in China – We are also interested in speaking with potential commercial / industrial partners to help take prototypes to the next level.
· Technical consultation
· Capital seeking


An innovative fundus camera with built-in AI for cost-effective detection and management of retinal diseases
Project background
Diabetic retinopathy (DR) is a complication of diabetes in the eye that can cause loss of sight if untreated, but a systematic screening program can almost eliminate sight loss by catching the disease early and giving treatment in time. Setting up such a programme is costly, and the demand for the number of trained health workers is immensely high. Our team has developed an innovative AI-enabled and low-cost camera that can image the retina at the back of the eye and provide diagnosis of DR with well-trained AI algorithms. This camera is well suited for low-cost DR screening programmes that could save the sight of millions of people.
Technology summary
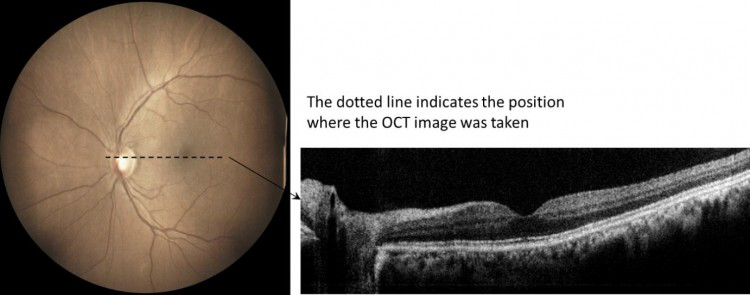
Our device prototype implements a novel optics design to integrate True Colour Fundus Camera (CFC) with an Optical Coherence Tomography (OCT). Both CFC and OCT share the same optics and optomechanics thus the overall system complexity and cost have been reduced. Our novel optical configuration provides an excellent field of view (60 degrees) retinal image without the need of pupil dilation. The accompanying OCT cross-sectional images provide additional information for significantly improved detection specificity. Together with our advanced AI algorithms, this camera system offers an ideal solution for diabetic retinopathy screening and the detection of early signs of many other eye diseases (e.g., glaucoma, age-related macular degeneration, central serous chorioretinopathy, macular hole, vitreomacular interface syndrome, and diabetic maculopathy).
Benefits/Advantages
Non-mydriatic exam: non-mydriatic fundus imaging exam eliminates the waiting time of both the eye drops effect and the exam itself, reduces the side effects, decreases the service length and the need of a companion for the patient. Multimodal imaging: our camera facilitates comprehensive diagnostics by combining multiple acquisition modes in a single device, including colour, IR and red-free fundus imaging modes and OCT cross-sectional imaging modes. True-colour confocal imaging: this particular technology facilitates diagnosis and monitoring of retinal diseases such as diabetic retinopathy, age-related macular degeneration and glaucoma. The unique combination of confocal imaging and white light illumination offers superior image quality and colour fidelity. Using white light, the retina appears as it looks when directly observed, as the entire visible spectrum is present in the captured image. Wide-field of view: Wide field optics allow imaging the central retina as well as the periphery. AI-enabled functions: our AI-enabled software provides added value to the device, including auto-focus, image enhancement, segmentation and visualisation, and accurate diagnosis functions (incl. automated DR grading and identification of macular lesion).
Market application
Our imaging technology is ideal for diabetic retinopathy screening, which will benefit over 100 million people with diabetes (PWD) in China alone and nearly 500 million PWD worldwide. The camera can be used in community centres and various hospitals and can also be used to manage eye diseases (e.g., age-related macular degeneration, glaucoma). Over 400 million people in China are aged 50 years or over, subject to various retinal diseases.
IP status
· In preparation
Ways of collaboration
· Various ways may be provided
· Technology commercialisation in China
· Capital seeking

Healthcare Technology Centre
Project background
The Healthcare Technology Centre is a proud partner of the £24 million pan-Wales Accelerate programme and supports the translation of promising ideas from the life science, health, and care sectors in Wales into new products, processes, and services, aiming to create long-lasting economic value alongside broader societal benefits. Our services complement those provided by our Accelerate partners and we work together to develop technology and innovation in the life sciences and health sectors in collaboration with NHS, industry, and academia. With a dedicated team of post-doctoral technologists, innovation and life science specialists, technicians, and project managers, alongside our start-of-the-art laboratory facilities, we are at the forefront of the technological development and adoption pipeline in Wales. The Healthcare Technology Centre has engaged with over 200 enterprises and has a portfolio of over 40 projects focussed on technology development within the life sciences and health sectors.
Technology summary
Range of technology projects and solutions, from sensors and devices, wellbeing technology and technology-enabled care, diagnostics, and analytics, digital and data.
Benefits/Advantages
Portfolio of over 40 projects focused on technology development Academic research expertise across a variety of life sciences and healthcare fields Long-term international collaborations sought Focus on creating sustainable innovation pipeline to improve regional health, wealth, and wellbeing.
Market application
Potential markets include NHS and health services both public and private internationally in addition to consumer and community markets also.
IP status
IP status depends on the specific project example and includes both projects with background IP and the potential to generate arising IP too.
Ways of collaboration
· Joint technical collaboration
· Technology commercialization in China
· Technical consultation
· Capital seeking

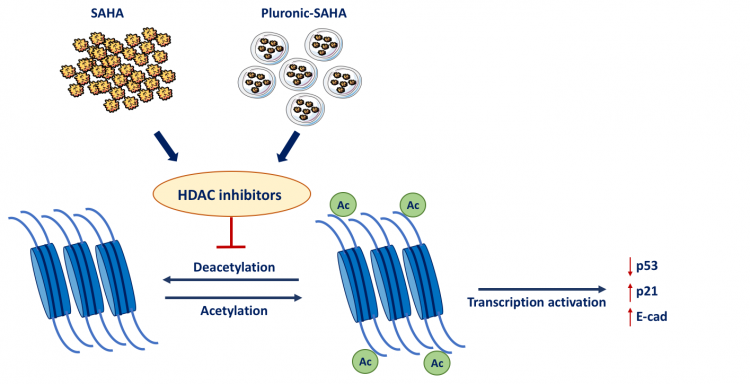
Nanomicelles potentiate histone deacetylase inhibitor efficacy in vitro
Project background
Amphiphilic block copolymers used as nanomicelle drug carriers can effectively overcome poor drug solubility and specificity issues. Hence, these platforms have a broad applicability in cancer treatment. We have developed Pluronic F127 nanomicelles containing the histone deacetylase inhibitor SAHA, which has an epigenetic-driven anti-cancer effect in several tumor types. SAHA-loaded nanomicelles were prepared using a thin-film drying method and characterized for size, surface charge, drug content, and drug release properties. Loaded particles were tested for in vitro activity and were effective on blocking cell cycle and markers of cancer progression.
Technology summary
Pluronic is a water-soluble amphiphilic molecule with a poly(oxyethylene)-block-poly (oxypropylene)-block-poly(oxyethylene) (PEOx–PPOy–PEOz) triblock structure (Farrugia et al. 2014), which self-assembles forming core–shell micelles in aqueous media. We have shown that the HDAC inhibitor SAHA can be efficiently loaded into pluronic F127 nanomicelles. We demonstrated that SAHA-loaded nanomicelles are able to efficiently release the drug in a time-dependent fashion. SAHA nanomicelles were shown to be more efficient than the free drug in reducing cell viability and inhibiting cell migration capacities of breast and cervical cancer cell lines, which represent two cancer types that still require more effective, epigenetic-based, treatments. Cellular uptake studies demonstrated the effective micellular uptake and intracellular distribution in a cell line-dependent fashion. In addition, the encapsulated SAHA remained effective in triggering cell cycle arrest and apoptosis in a dosage-dependent manner. The HDAC inhibitor also altered the expression of the EMT markers E-cadherin and N-cadherin, suggesting that effective delivery has the potential to reverse the aggressive, metastatic phenotype of these cancer models.
Benefits/Advantages
Encapsulation of SAHA into nanomicelles enhances the potency of this epigenetic drug in breast and cervical cancer cell models This effective formulation could enhance drug delivery to tumor sites, and overcome current issues in delivering HDAC inhibitors to solid tumors, while also reducing side-effects associated with systemic delivery of the free drug These effects are likely to be specific to different cancer types, as we found that the SAHA-loaded nanomicelles displayed different uptake rates, and directed intracellular trafficking in the two different cancer cell models.
Market application
As SAHA has been shown to cause harmful side-effects, encapsulation could be an effective route to reducing systemic toxicity Encapsulation could result in the use of less drug while still obtaining the required therapeutic effect, or more effective Encapsulation could result in tumor site specific delivery and uptake due to the inherent properties of nanostructures.
Ways of collaboration
· Joint technical collaboration. To date this project was a collaboration with Dr Chao Li at XJTU Nanoscience Academy Suzhou.

Antibody Drug Conjugate targeting the RAGE receptor protein
Project background
The Receptor for Advanced Glycation End products (RAGE) is a type I cell-surface transmembrane receptor member of the immunoglobulin superfamily, and has been observed to show increased expression in certain cancers. RAGE expression in healthy tissue is in general absent or very low with the exception of lung, making it an ideal target for ADC therapy. As proof of concept, in vitro and in vivo studies confirmed the toxicity and specificity of our ADCs for ovarian and endometrial cancer cells. Additionally, our ADCs are more efficacious in vitro than an ADC equivalent to the clinically approved ADC, Kadcyla®.
Technology summary
We have significantly advanced the pre-clinical development of our ADCs by using a proof-of-concept IgG2-ADC and undertaking in vivo and ex vivo distribution and toxicology studies. The successful completion of this initial project has confirmed that (1) our ADCs have no systemic toxicity confirming previous observations that the target is non-essential to life, (2) The tissue distribution profile following intravenous administration of the ADC, confirms it accumulates in the tumour of tumour-bearing animals and in the reproductive organs (endometrium and ovaries) of non-tumour and tumour-bearing animals, (3) the ADC molecules has the ability to penetrate tumours and reduce the effectively reduce the tumour volume when tested in a xenograft model of endometrial cancer (in vivo animal study) (J Immunother Cancer.2019 Oct 29;7(1):280. doi: 10.1186/s40425-019-0765-z).
Benefits/Advantages
Antibody Drug conjugates (ADCs) are among the first generation of targeted therapies with the potential to transform cancer treatment. These novel therapeutic agents are armed antibodies with lethal cytotoxin payloads targeting cancer cells while sparing healthy cells and reducing side effects. These target-seeking molecular missiles have proven very effective in oncology applications, and are exemplified by Adcentris and Kadcyla, currently in the market. To date, a total of ten ADCs have been approved by the FDA, of which 6 have been approved in the last 18th months. Aiming to develop novel therapeutics against these gynaecological cancers, we have identified a novel target for ovarian and endometrial cancer therapies and developed a therapeutic modality based on antibody drug conjugates (ADCs).
Market application
At a similar cost to Avastin, £25,000 for a 7-month course, RAGE-ADC this could translate to a market of £177 million in the UK alone, and around £1.6Bn in Europe, with an expected higher market in Asia. Even if the drug were restricted to those who presented with advanced disease, this represents around 80% of all new diagnoses, due to the absence of symptoms in early-stage disease of disease. This represent a strong clinical and commercial case for development of a novel RAGE antibody drug conjugate targeting ovarian cancer. The potential market value of an ADC is given by the uniqueness of its target. The ADC market is avid of new molecules as evidenced by the increased amount of partnership and licensing agreements. We aim to approach major ADC development companies as our engagement strategy for the RAGE ADC programme.
IP status
Granted as EP3209694, US 10,406,124, and pending applications CA 2963744 and US 16/541,107
Ways of collaboration
· Joint technical collaboration
· Technology commercialization in China
· Capital seeking

CAPTURE (Circular APplications To Utilise and Retain Energy)
Project background
CAPTURE is an interdisciplinary project centred at Swansea University dedicated to the development of a circular approach to the manufacture and management of electrochemical energy storage solutions, particularly next-generation batteries. The research philosophy is based on a ground-up approach, where all energy storage components (material, processes, management) are conceptualised , designed and developed from incept within a circular economy framework , ensuring an optimum use of resources, re-use and recyclability of its components towards a zero-carbon approach.
Technology summary
With petrol and diesel engines making up 73% of transport-related pollution switching to electric vehicles is key to driving the decarbonisation of road transport and mitigating one of the main contributors to our warming climate. A key element of this transition is energy storage and the capture of energy that can be harnessed at another point in time. To date lithium-ion batteries have enabled the switch to electric vehicles and the portable electronics revolution, with other applications continuing to grow, including “behind-the-meter” residential energy storage systems and integration with the grid. However, with energy density having plateaued in standard LIBs, new manufacturing solutions are needed to meet demand and keep pace with increasingly power-hungry applications by unlocking the commercial potential of other energy storage technologies including those based on Lithium/Sodium metal anodes and Llithium/Sulphur batteries which promise a large increase in energy density. However large-scale production of these next-generation batteries is currently stalled by technical and manufacturing limitations. CAPTURE aims to tackle these issues by delivering a step-change in the manufacturing of next-generaton battery technologies by providing scalable and sustainable solutions. This is achieved by leveraging on (i) state-of-the-art fabrication and testing equipment including pouch and cylindrical cells production lines, (ii) a wide platform of industry partners from materials providers to battery manufactureres and (iii) new and unique multidisciplinary collaboration assembled specifically to overcome the challenges associated scalable and sustainable manufacturing, bringing together academic expertise from across multiple engineering disciplines.
Benefits/Advantages
Building on fundamental work to determine the conditions and pre-requisites necessary to achieve high-energy density and long-cycle life batteries, CAPTURE offers a new approach using unique commercial-scale equipment for fabrication/testing and enabling the integration into existing production lines. The implications of the research programme for research and development are multifaceted, impacting researchers and industries working on innovative energy storage solutions based on Li/Na-metal (e.g. lithium-air, Li-S and solid-state batteries) as well as battery integration and e-drive technologies. Furthermore, the toolbox of ex-situ and in-situ characterisation techniques developed will impact the production of high-performance electrode active materials, scaling-up processes for electrode manufacturing and in-line optimisation of battery components.
Market application
Through advancements in the sustainable manufacturing of rechargeable batteries, which are rooted within many technologies (transport, mobile tech, portable equipment, grid storage etc.) and with prospects in emerging sectors (heavy-freight, storage of renewably generated electricity, electric aviation), keeping at the forfront of technological development in this domain has huge benefits for various market applications. Primarily , CAPTURE aims to address the needs of industries focusing on the production of batteries at cell and pack level.
Ways of collaboration
· Joint technical collaboration
· Technology commercialization in China